ciclesonide
Boehringer Ingelheim Vetmedica GmbH
55216 Ingelheim/Rhein
Germany
Fareva Amboise
Zone Industrielle
29 Route des Industries
37530 Pocé-sur-Cisse
France
Each actuation (ex nostril adapter) contains:
Active substance:
Ciclesonide 343 micrograms
Excipients:
Ethanol 7.9 mg
Clear, colourless to yellowish solution.

For the alleviation of clinical signs of severe equine asthma (formerly known as Recurrent Airway Obstruction– (RAO), Summer Pasture Associated Recurrent Airway Obstruction – (SPA-RAO)).

Do not use in known cases of hypersensitivity to the active substance, to corticosteroids or to any of the excipients.

Mild nasal discharge was commonly observed during safety and clinical studies.
The frequency of adverse reactions is defined using the following convention:
If you notice any side effects, even those not already listed in this package leaflet or you think that the medicine has not worked, please inform your veterinary surgeon.

Horse

Inhalation use.
The number of actuations to be administered is the same for all horses.
The total treatment duration is 10 days:
Day 1 to 5:
8 actuations (corresponding to 2,744 μg ciclesonide) administered twice daily approximately 12 h apart.
Day 6 to 10:
12 actuations (corresponding to 4,116 μg ciclesonide) administered once daily approximately 24 h apart.
The onset of clinical improvement may take several days. The 10 days treatment schedule should normally be completed. In case of any concerns related to the treatment the responsible veterinarian should be consulted.
The Aservo® EquiHaler® contains sufficient inhalation solution for one horse for the entire treatment duration of the 10 days and an additional amount covering priming and potential losses during administration.
Treatment schedule for use:
| Treatment days 1 to 5 |
Treatment days 6 to 10 |
| 8 actuations morning and evening approximately 12 h apart | 12 actuations once daily approximately 24 h apart |

The "Instructions for handling and use of the Aservo® EquiHaler®" is provided in section "Other information" of this leaflet.

Meat and offal: 18 days.
Not authorised for use in horses producing milk for human consumption.

Keep out of the sight and reach of children.
This veterinary medicinal product does not require any special storage conditions.
Shelf life after first activation: 12 days.
Do not use this veterinary medicinal product after the expiry date which is stated on the carton and the label after EXP.

Special care should be taken when administering the veterinary medicinal product. To ensure an efficacious administration, the breath indicator in the chamber wall of the nostril adapter needs to be observed: when the horse inhales, the membrane of the breath indicator curves inwards. During exhalation, the membrane of the breath indicatior curves outwards. The spray should be released at the beginning of inhalation, i.e. when the breath indicator starts curving into the chamber. If the movement of the breath indicator cannot be observed, assure the correct positioning of the nostril adapter. If movement of the breath indicator is still not visible or the movement is too rapid, the product should not be administered. Efficacy of the product has not been established in horses with acute exacerbations (<14 days duration) of clinical signs.

Safety of the veterinary medicinal product has not been established in horses weighing less than 200 kg body weight, or in foals.
The prescribing veterinarian should assess if the horse has a temperament suitable for a safe and efficacious administration of the Aservo® EquiHaler® in agreement with good veterinary practice. Horses might not adapt to an easy and safe application of the Aservo® EquiHaler® within a couple of days. An alternative treatment should be considered if the horse does not adapt to the treatment with Aservo® EquiHaler®.
The onset of clinical improvement may take several days. The use of concomitant medication (such as bronchodilators) and environmental control may need to be considered in cases of severe clinical signs of respiratory obstruction, at the discretion of the attending veterinarian.

Follow closely the instructions for handling and use of the Aservo® EquiHaler® as provided in the package leaflet section “Other Information”.
Acclimatising the horse with a training device, available from the marketing authorisation holder, prior to treatment start has shown to ease the administration of the veterinary medicinal product. Surveillance has shown cases where the horses were non-cooperative and therefore could not be treated according to the product information. In case a horse has a tendency towards defensive behavioural reactions, additional safety precautions could be considered (e.g. employ a second person to handle the horse).
Administration of the product should take place in well ventilated surroundings.
People with known hypersensitivity to ciclesonide or any of the excipients should avoid contact with the veterinary medicinal product.
Inhalative and intranasal corticosteroids may cause rhinitis, nasal discomfort, nosebleed, upper respiratory tract infection and headache. An aerosol filtering mask must be worn during handling and administration. This prevents inadvertent inhalation in case of unintended release of actuations outside the nostril or without the nostril adapter.
The product can cause irritation to the eyes due to its ethanol content. Avoid contact with eyes. In case of accidental eye contact, rinse with large quantities of water.
In case of experiencing an adverse reaction due to accidental inhalation, and in case of eye irritation, seek medical advice and show the package leaflet or the label to the physician.
These precautions should be followed by the person administering the product and persons in close proximity to the horse’s head during administration.
The safety of ciclesonide after inhalatory exposure has not been established in pregnant women. In animal studies ciclesonide has been shown to induce malformations in foetuses (cleft palate, skeletal malformations). Pregnant women should therefore not administer the product.
If the Aservo® EquiHaler®
is visually damaged it should not be used any more.It is essential to keep the product out of reach for children.

The safety of the veterinary medicinal product has not been established during pregnancy or lactation.
Use only according to the benefit/risk assessment by the responsible veterinarian.
The product was shown to be teratogenic following oral administration after high doses in rabbits but not in rats.

Concomitant use of clenbuterol in a field study in seven horses with severe equine asthma did not indicate any safety concerns.

After administration of the veterinary medicinal product at up to the 3-fold recommended dose for 3 times the recommended treatment duration no relevant clinical signs could be observed.

Medicines should not be disposed via wastewater or household waste. Ask your veterinary surgeon how to dispose of medicines no longer required. These measures should help to protect the environment. The cartridge contains residual amount of the product at the end of the course of administration. This should be taken into account at disposal of the used veterinary medicinal product.

January 2023
Find more product information by searching for the ‘Product Information Database’ or ‘PID’ on www.gov.uk

Pack size: One Aservo® EquiHaler® with a nostril adapter and a pre-inserted cartridge. The cartridge contains sufficient inhalation solution for the entire treatment duration (140 treatment actuations) and an additional amount covering priming and potential losses during administration within the 10 day treatment duration. Additionally there is residual solution which cannot be delivered with the required accuracy, and should therefore not be administered. The cartridge cannot be removed from the Aservo® EquiHaler®.
For animal treatment. To be supplied only on veterinary prescription.

Please read the following instructions carefully prior to first use of the Aservo® EquiHaler® which can be also found when using the URL info.equi-haler.com or the enclosed QR code. The Aservo® EquiHaler® is a product for inhalation for horses. The Aservo® EquiHaler® contains sufficient inhalation solution for one horse for the entire duration of the 10 days treatment and an additional amount covering priming and potential losses during administration.

The Aservo® EquiHaler® is for left hand use only. While holding the Aservo® EquiHaler® with your left hand, you hold and control your horse with your right hand.

Remove the Aservo® EquiHaler® from the outer carton.
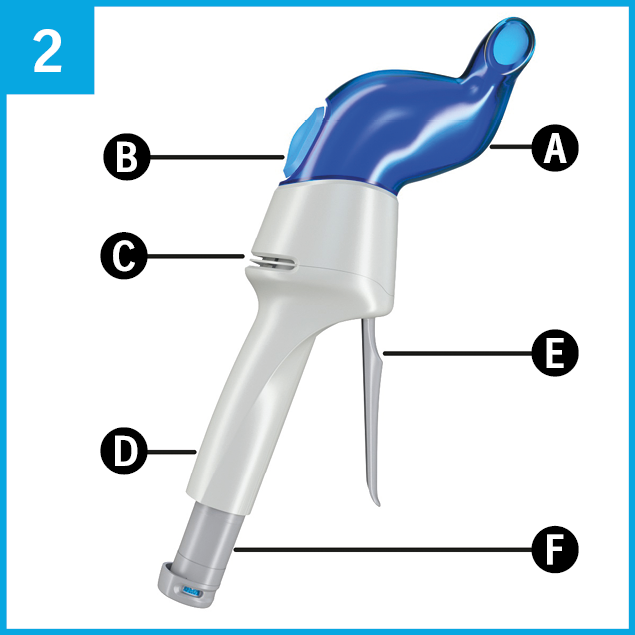
Familiarise yourself with the Aservo® EquiHaler®.
It consists of:
A Nostril adapter
B Breath indicator
C Air inlet
D Handle
E Prime and release lever
F Piercing element with fill indicator
In the following chapters, activation, priming, administration, cleaning, and storage of the Aservo® EquiHaler® is described in detail.

Activation of the Aservo® EquiHaler® has to be performed only once prior to first use.

To activate the Aservo® EquiHaler®, the piercing element F needs to be inserted into the handle of the inhaler without pressing the lever E.
Put your right hand under the dark grey piercing element F…

... and push the piercing element F up completely into the handle D until you will hear a click.
In the final position, the piercing element will disappear entirely in the handle D and is no longer visible.
The Aservo® EquiHaler® is now activated, but not ready to use.
An aerosol filtering mask must be worn during handling and administration. This prevents inadvertent inhalation in case of unintended release of actuations outside the nostril or without the nostril adapter.

Priming is required to ensure accurate initial dosing. Priming is performed only once and comprises three (3) actuations (see below). The spray will be fully visible after the third actuation.
When pressing the lever E of the Aservo® EquiHaler® for the first time, the lower part of the piercing element with the fill indicator F will become visible again.
Do not push the piercing element back up into the device.

The Aservo® EquiHaler® is designed for the left hand use only and for use in the left nostril of the horse only. While holding and operating the Aservo® EquiHaler® with your left hand, you hold and control your horse with your right hand.
Each actuation consists of the following two steps (pictures 5 to 8):

Hold the Aservo® EquiHaler® upright in your left hand.

Step 1:
Press the prime and release lever E until it touches the handle and a click can be heard.
Release the lever E allowing it to slide back into its starting position.

The display of the fill indicator in the piercing element is partially covered with a red flap.

Step 2:
Press the prime and release lever E again with light pressure only until you hear an audible click. Let the lever slide back into its starting position. The spray will be released subsequently into the nostril adapter A.
The fill indicator now displays the filling level in %.
Once activated the product should no longer be used after 12 days.
Please note:
Should the piercing element be accidently pushed completely into the handle again, it will automatically slide into the correct position when the Aservo® EquiHaler® is actuated the next time.

The nostril adapter should remain in the nostril during the entire administration of the 8 or 12 actuations. If the nostril adapter slides out of the nostril during administration please re-insert into the nostril again.
The Aservo® EquiHaler® should be administered in a well ventilated area.

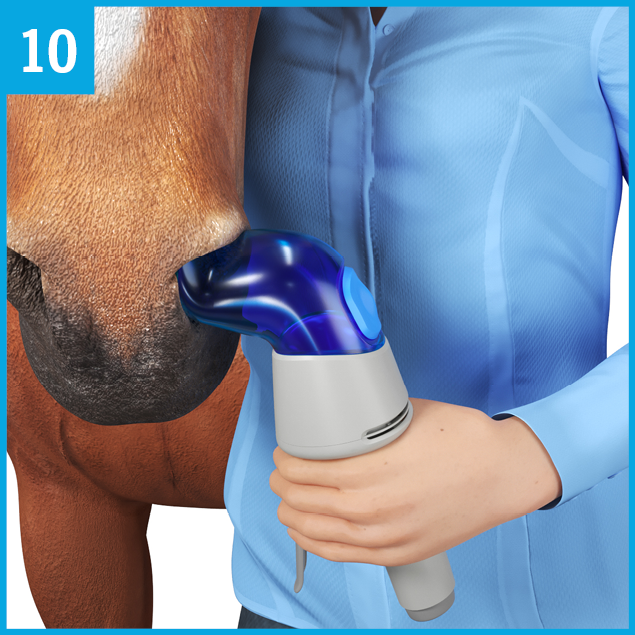
Hold the Aservo® EquiHaler® in your left hand. Make sure that the air inlet C is not obstructed.
Stand on the left side of the horse so that the horse’s head is next to your right shoulder.
Insert the nostril adapter A coming from a horizontal position carefully into the horse’s left nostril, and gently rotate the Aservo® EquiHaler® ...
... into an upright position. Assure that the nostril adapter is inserted in the nasal cavity.
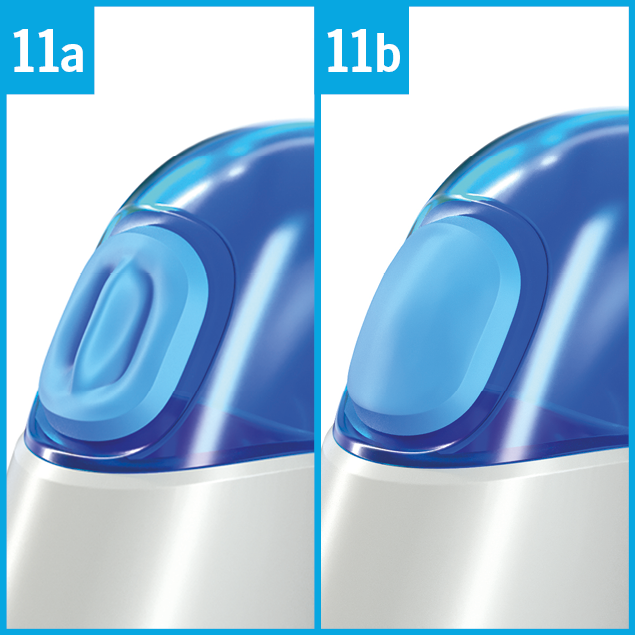
Observe the movement of the breath indicator B :
When the horse inhales, the membrane of the breath indicator curves inwards (picture 11a).
When the horse exhales, the membrane of the breath indicator curves outwards (picture 11b).
The optimum time for release is at the beginning of the horse ́s inspiration when the breath indicator B begins to curve inwards.
Please note: In order for the breath indicator to demonstrate when the horse inhales or exhales, the nostril adapter A must be correctly placed in the nostril and should fit tightly.
If the movement of the breath indicator cannot be observed, assure the correct positioning of the nostril adapter. If still no movement is visible, the product should not be administered.
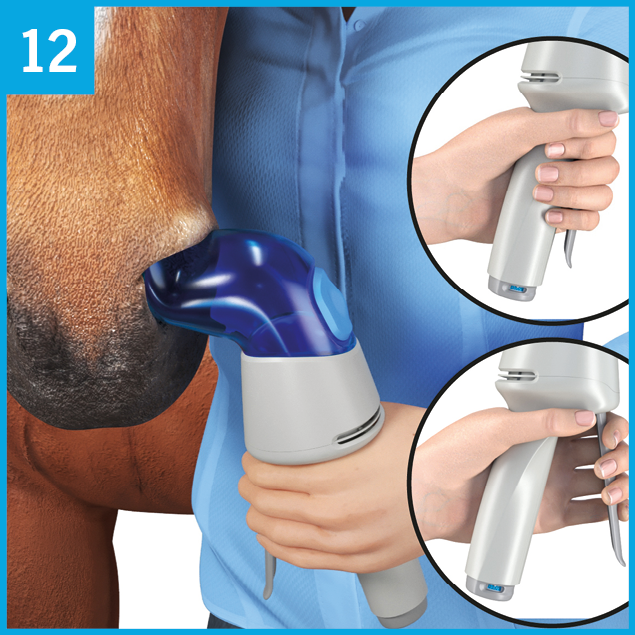
Every actuation should be performed following the two steps explained in pictures 6, 7, and 8.
Administer the correct number of actuations as described in section “Dosage, route and method of administration”, see above.

The fill indicator shows the percentage of actuations available in the inhaler. The fill indicator should display 100% prior to first use, i.e. after the Aservo® EquiHaler® is primed.

The display of the fill indicator only moves after several actuations.
After administration of the 10 days treatment schedule the display has reached the position 0%.

The product allows an additional amount of actuations covering potential losses during administration. In this case the display of the fill indicator moves further and stops at the horse head. The inhaler must not be used after the fill indicator has reached the horse head.


After each use and before cleaning, check that the fill indicator is blue/white. If it is red, press the prime and release lever E until the click is heard. This will assure that you do not accidentially release any spray. To avoid inhalation, hold the inhaler away from your body.

After use, twist and lift nostril adapter A from the handle D.
Store the handle in a clean and dry place.

Rinse the nostril adapter A only in clean running water. Do not use any brushes or cleaning products.
The handle can be carefully wiped with a moist cloth.
The Aservo® EquiHaler® is not suitable for the dishwasher.

The nostril adapter A must be air dried in an upright position for at least 4 hours.
Do not rub dry or heat.
Do not use technical equipment such as hair dryer, microwave, or heating element.

Once the nostril adapter A is dry it should be reattached to the handle D by pushing it down firmly and twisting slightly until it slides into its place.
The nostril adapter A only locks in one position and should fit tightly to the handle.
Gently pulling up on the nostril adapter after attaching to the handle should reveal the nostril adapter is firmly attached.
The Aservo® EquiHaler® is now ready for the next use.

This veterinary medicinal product does not require any special storage conditions.
Do not store the Aservo® EquiHaler® if the fill indicator is partially covered with a red flap.