Corticosteroid

Aservo™ EquiHaler™ is a non-pressurized metered dose inhaler that provides an aerosolized mist and drug cartridge combination containing a solution of 30 mg/mL of the prodrug ciclesonide. The prodrug ciclesonide is enzymatically converted to the pharmacologically active metabolite desisobutyryl-ciclesonide (des-ciclesonide) following inhalation.

For the management of clinical signs associated with severe equine asthma in horses.

Ciclesonide inhalation solution, 343 mcg per actuation.

For intranasal inhalation only. Aservo™ EquiHaler™ may be used as part of the overall management of severe equine asthma in addition to other appropriate strategies to control clinical signs, such as environmental changes and bronchodilator therapy, as needed.
The initial dose of Aservo™ EquiHaler™ is 8 actuations (2 744 mcg ciclesonide) twice daily for 5 days, followed by 12 actuations (4 116 mcg ciclesonide) once daily for 5 days.
| Total treatment duration is 10 days | |
| Treatment days 1 to 5 |
Treatment days 6 to 10 |
| 8 actuations/puffs Twice Daily |
12 actuations/puffs Once Daily |
Prior to use, please refer to the detailed instructions for handling and use of the Aservo™ EquiHaler™ provided in the “User Manual”.
Aservo™ EquiHaler™ should only be administered in the left nostril of the horse. Following careful and proper insertion, the Aservo™ EquiHaler™ should have a snug fit in the left nostril. A snug fit is required to ensure the horse receives a complete dose of Aservo™ EquiHaler™.

Do not use in horses with hypersensitivity to ciclesonide or corticosteroids.

Safe use of Aservo™ EquiHaler™ has not been evaluated in pregnant or lactating mares. Clinical and experimental data have demonstrated that corticosteroids administered orally or by injection to animals may induce the first stage of parturition if used during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta, and metritis. Additionally, corticosteroids administered during pregnancy can be teratogenic. Therefore, Aservo™ EquiHaler™ should only be used in pregnant mares if the potential benefit justifies the potential risk to the fetus.
Administration of corticosteroids may worsen existing bacterial, fungal, or viral infection. Secondary infections should be ruled out before prescribing Aservo™ EquiHaler™. Inhaled corticosteroids should be used with caution, if at all, in horses with active infection of the respiratory tract. If clinical signs do not improve or worsen despite administration of Aservo™ EquiHaler™, the prescriber should consider additional diagnostic evaluation.
Due to the potential for exacerbation of clinical signs of laminitis, glucocorticoids should be used with caution in horses with a history of laminitis, or horses otherwise at a higher risk for laminitis.
Use with caution in horses with chronic nephritis, equine pituitary pars intermedia dysfunction (PPID), and congestive heart failure.
Concurrent use of other anti-inflammatory drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs) or other corticosteroids, should be approached with caution. Due to the potential for systemic exposure, concomitant use of NSAIDs and corticosteroids may increase the risk of gastrointestinal, renal, and other toxicity. Consider appropriate wash out times prior to administering additional NSAIDs or corticosteroids.
Warnings: Keep out of the reach of children. Not for use in humans. In case of accidental inhalation, seek medical advice immediately and show the package insert or the product label to the physician. People with known hypersensitivity to ciclesonide or any of the excipients should avoid contact with Aservo™ EquiHaler™.
Do not use in horses intended for food.

The clinical safety of Aservo™ EquiHaler™ was assessed in a vehiclecontrolled (the identical device containing the vehicle with no ciclesonide), randomized, double-blind 10-day field study followed by a 90-day open-label, extended-use field safety phase. In this field study, 319 client- or university-owned horses received either Aservo™ EquiHaler™ or vehicle control during Phase 1. Following Phase 1, 108 horses were enrolled in Phase 2, during which they could have received treatment for up to an additional 3 months. All horses in Phase 2 received the Aservo™ EquiHaler™. Three hundred nineteen horses (163 ciclesonide and 156 control) of various breeds, 6 to 34 years of age, and weighing approximately 204 kg to 839 kg were included in the field-study safety analysis.
Adverse reactions that were reported in Phase 1 are listed in Table 1, and adverse reactions that were reported in Phase 2 are listed in Table 2. The most common adverse reaction reported was coughing either during or immediately following inhalation (both Aservo™ EquiHaler™ and vehicle control groups).
Leukocytosis and/or neutrophilia developed in Phase 1 in several horses in both treatment groups, and in eight horses in Phase 2. Some of these horses also had concurrent clinical signs of infection, such as fever, which may have contributed to the leukocytosis.
One Aservo™ EquiHaler™-treated horse developed fever and leukocytosis characterized by a mature neutrophilia on Day 10 that was not present at screening. Other horses had possible alternate explanations for a stress leukogram, such as pain from a hoof abscess or poor acceptance of the device which may have caused stress from the study procedures.
In Phase 1, three Aservo™ EquiHaler™-treated horses had increases in serum sorbitol dehydrogenase (SDH) above the reference range on Day 10 (high values were 12.4, 18.1 and 30.4 U/L); their baseline values were within the reference range (2-6 U/L). None of these horses had clinical signs attributable to elevated SDH levels. One horse also had an increase in serum gamma-glutamyltransferase (GGT) compared to baseline, but the GGT value at baseline was already above the reference range. By Day 40 of Phase 2, and again when measured at Day 100, this horse’s SDH and GGT values were within normal limits. In Phase 2, one horse developed an elevation in SDH (63.8 U/L) following the 6th course of Aservo™ EquiHaler™ treatment. This horse had elevations in alkaline phosphatase (269 U/L, reference range 76-262 U/L), aspartate aminotransferase (511 U/L, reference range 194-431 U/L), and GGT (128 U/L, reference range 9-37 U/L). Reported adverse events in this horse at various time points during the study included coughing, nasal discharge, tachycardia, and hyperthermia. After the visit at which abnormal blood work was detected, the owner opted to withdraw the horse from the study (approximately Day 75).
In Phase 1, one horse developed severe hives 30 minutes after the fourth dose of Aservo™ EquiHaler™. This horse was removed from the study due to this adverse reaction.
Table 1: Number (%) of Horses with Adverse Reactions in Phase 1 of field study
| Adverse Reaction | Aservo™ EquiHaler™ (N=163) n (%) |
Vehicle Control (N=156) n (%) |
|---|---|---|
| Cough | 27 (16.6) | 27 (17.3) |
| Nasal Discharge | 17 (10.4) | 17 (10.9) |
| Leukocytosis and/or neutrophilia | 10 (6.1) | 7 (4.5) |
| Sneezing | 5 (3.1) | 3 (1.9) |
| Nasal irritation/bleeding | 2 (1.2) | 3 (1.9) |
| Increase in serum Sorbitol Dehydrogenase (SDH) | 3 (1.8) | 0 (0) |
| Hives | 1 (0.6) | 0 (0) |
*Horses may have had one or more reports of given adverse reaction
Table 2: Number (%) of Horses with Adverse Reactions in Phase 2 of field study
| Adverse Reactions | Aservo™ EquiHaler™ (N=100) n (%) |
|---|---|
| Cough | 12 (12) |
| Nasal Discharge | 13 (13) |
| Leukocytosis and/or neutrophilia | 8 (8) |
| Nasal irritation/bleeding | 6 (6) |
| Laminitis | 3 (3) |
| Sneezing | 2 (2) |
| Increase in serum Sorbitol Dehydrogenase (SDH) | 1 (1) |
*Horses may have had one or more reports of given adverse reaction
Laminitis was reported in three horses during Phase 2. Two of the horses were noted to have a history of or physical evidence of prior laminitic episodes. The third horse had no prior history of laminitis, was initially treated for thrush, and responded to conservative therapy within a few weeks. No definitive diagnosis of laminitis was made, and the examining veterinarian concluded the horse’s environment played a role in the development of clinical signs.
In Phase 1, in both treatment groups, nasal soreness, bleeding, scabs in the nostril, and redness of the nostril were reported in five horses. In Phase 2, horses were reported to have epistaxis (2 horses), blood tinged discharge (1 horse), nose bloody and raw (1 horse), sensitive nostril (1 horse), and bright pink color of nasal mucus membranes (1 horse). One case of epistaxis was reported 9 days after the last dose was administered, and one case was reported from the right nostril less than 24 hours following the last dose. The blood tinged discharge from the left nostril was reported 11 days following the last dose administered. In Tables 1 and 2, these similar clinical signs are summarized as nasal irritation/bleeding.

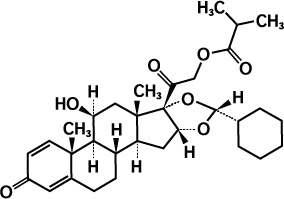
Ciclesonide is a prodrug, which is enzymatically converted to the pharmacologically active metabolite desisobutyryl-ciclesonide (desciclesonide) following inhalation. The glucocorticoid-receptor affinity of des-ciclesonide is 120 times greater than the parent compound’s affinity and 12 times greater than dexamethasone’s affinity.
Des-ciclesonide anti-inflammatory properties which are exerted via a wide range of inhibitory activities against cell types including mast cells, eosinophils, basophils, lymphocytes, macrophages, and neutrophils as well as against pro-inflammatory mediators such as histamine, eicosanoids, leukotrienes, and cytokines. The structural formula for ciclesonide is:
A four period pharmacokinetic study was conducted using 6 average weight horses and 6 light weight horses. Each horse received the following dosing regimens:
Period 1: 12 actuations (4 116 mcg ciclesonide/horse) as a single inhalation administration,
Period 2: 16 actuations (5 488 mcg ciclesonide/horse) as a single inhalation administration,
Period 3: 8 actuations (2 744 mcg ciclesonide/horse) twice a day for 4 consecutive days and once on the fifth day,
Period 4: 8 actuations (2 744 mcg ciclesonide/horse) twice a day for 5 consecutive days followed by 12 actuations (4 116 mcg ciclesonide/horse) once a day for 5 days.
Blood samples were collected at pre-dose, 0 minutes, 5 minutes, 15 minutes, 30 minutes, and 1, 2, 4, 6, 8, 10, 12, and 24 hours after a single inhalation administration of 2 744, 4 116, and 5 488 mcg in periods 1, 2, 3, and after multiple doses of 2 744 and 4 116 mcg in Periods 3 and 4.
Urine samples were collected at 24 and 48 hours in Period 4. Plasma and urine samples were analyzed for ciclesonide and des-ciclesonide concentrations using validated liquid chromatograph/ mass spectrometry methods.
Ciclesonide was rapidly metabolized to des-ciclesonide; there were quantifiable concentrations of des-ciclesonide in most animals at 5 minutes post-dose. Urine concentrations of ciclesonide and desciclesonide were below the limit of quantification at 24 and 48 hours after 2 744 mcg twice a day for 5 days followed by 4 116 mcg once a day for 5 days. Table 3 summarizes the results of the pharmacokinetic analysis for des-ciclesonide in average and light weight horses (adjusted for body weight) after inhalations of 2 744 mcg ciclesonide twice a day for 5 days followed by inhalations of 4 116 mcg ciclesonide once a day for 5 days.
Table 3: Mean (± standard deviation; SD) pharmacokinetic parameters of des-ciclesonide after inhalations of 2 744 mcg ciclesonide twice a day for 5 days followed by inhalations of 4 116 mcg ciclesonide once a day for 5 days
| Parameter | Dose (mcg) |
Average Horse | Light Horse |
| Cmax (pg/mL) | 2744 | 232.67 (40.89) | 297.00 (92.0) |
| Tmax (hrs)† | 2744 | 0.50 (0.5-0.5) | 0.50 (0.25-1.0) |
| AUClast (hr*pg/mL) | 2744 | 824.26 (81.77) | 1087.07 (137.85) |
| t ½ (hrs) | 2744 | 5.00 (1.06) | 5.94 (2.12) |
| Cmax (pg/mL) | 4116 | 297.50 (104.62) | 493.00 (248.27) |
| Tmax (hrs)† | 4116 | 0.50 (0.5-1.0) | 0.50 (0.5-1.0) |
| AUClast (hr*pg/mL) | 4116 | 1011.39 (291.72) | 1550.31 (690.02) |
| t ½ (hrs) | 4116 | 6.08 (2.42) | 9.75 (4.20) |
Cmax = maximum plasma concentration
†Tmax = time to maximum concentration; median (range)
AUClast = area under the concentration vs time curve to the last quantifiable concentration
t ½ = half-life
The drug exposure of des-ciclesonide was higher and more variable in the lightweight horses compared to the average weight horses. For des-ciclesonide in both average and light weight horses, there was a greater than dose proportional increase in Cmax and AUClast with an increase in dose from 2 744 to 4 166 mcg, after a single dose. There was minimal accumulation of des-ciclesonide after inhalations of 2 744 mcg ciclesonide twice a day for 5 days followed by inhalations of 4 116 mcg ciclesonide once a day for 5 days.

Animal Safety: In a 1-month target animal safety study, 32 healthy adult horses (16 mares and 16 geldings) were assigned to 1 of 4 treatment groups, with 8 horses per group. Each treatment group contained 6 average weight horses (371.9 to 539.8 kg; mean 477.0 kg) and 2 light weight horses (121.5 to 260.5 kg; mean 179.5 kg).
Light weight horses were specifically included to assess the safety of the highest mg ciclesonide/kg body weight dose.
Horses were randomized into four treatment groups, each receiving a different multiple of the therapeutic dose for 30 days (three times the treatment duration) administered with the nasal inhaler device. The treatment groups received 0X (vehicle control), 1X, 2X, and 3X the therapeutic dose of ciclesonide, respectively. Each treatment group included a total of 8 horses, six average weight horses (3 males and 3 females) and two light weight horses (1 male and 1 female).
Horses in the control group received the same number of actuations as the highest dose group (3X) from the nasal inhaler device with vehicle only.
Mild mucoid to seromucoid nasal discharge was observed in all treatment groups, including controls, and did not appear to increase after treatment. In general, for the 0X, 2X, and 3X groups, the number of different fungal species isolated from the nares increased by Day 30; however, the result may have been an effect of extended time in the barn with direct contact with other horses and the environment rather than related to treatment with the inhaler. One horse cultured Candida species post-treatment, and that horse had no abnormal clinical signs. The dosing was intended to mimic twice daily dosing for the first 15 days, and then the once daily dosing for the second half of the study.
Facial asymmetry or deviation of the muzzle was observed in two horses during the study in the ciclesonide-treated groups. The signs resolved in one horse prior to the study conclusion. No other related abnormal signs were observed, and no pathologic findings were correlated with this abnormality.
Cortisol levels were measured as a marker for suppression of the hypothalamic-pituitary-adrenal axis by systemic action of corticosteroids. Two light weight horses in the 3X ciclesonide-treatment group had values (16.3 and 14.1 ng/mL) that were lower than any other values recorded in the study (including baseline values and values from control horses), and lower than the normal reference range (27.6-73.2 ng/mL) on Day 14 (the end of the twice daily treatment period). At Day 29, one horse’s cortisol value returned to within the reference range (55.9 ng/mL) and the other remained low (15.9 ng/mL). These reductions in cortisol values in the smallest horses in the highest dose group may represent cortisol suppression from corticosteroid administration; however, neither of these horses had adverse events attributable to administration of ciclesonide.
Sorbitol dehydrogenase (SDH) levels were not statistically significantly different between ciclesonide treatment groups and the control group. However, one control horse (Day 14, SDH 13.6 U/L), one light weight horse in the 2X treatment group (Day 29, SDH 9.3 U/L), and two light weight horses in the 3X treatment group (Day 29, SDH values of 10.4 and 8.8 U/L) had SDH values that were above the reference range (reference range 1-8 U/L). These values did not correspond with increases in other liver enzymes and the horses remained clinically normal.

In a multi-center, vehicle-controlled, randomized, double-blind, field study, 320 horses were randomly allocated to receive either Aservo™ EquiHaler™, the investigational veterinary product containing the active ingredient ciclesonide, or the control product, consisting of an identical inhaler device containing the vehicle with no ciclesonide. The study consisted of two phases, and 108 of the 320 enrolled horses participated in both Phase 1 and Phase 2 of the study.
Phase 1 of the study consisted of a double-blind, 10-day evaluation of the effectiveness and safety of Aservo™ EquiHaler™ compared to vehicle control for the management of clinical signs associated with severe equine asthma in both client- and university-owned horses under field conditions. Modifications of the horse’s environment were not allowed during Phase 1. Following completion of Phase 1, 108 horses participated in the 90-day open-label Phase 2 to evaluate the safety of Aservo™ EquiHaler™ during repeated use.
In Phase 1, a weighted clinical score (WCS) was calculated to assess both eligibility at study enrollment and treatment success at the conclusion of the study.
The WCS was comprised of nine clinical parameters (respiratory rate, nasal discharge, nasal flaring, abdominal lift, tracheal sounds, bronchial tones, crackles, wheeze, and cough) with a maximum score of 23. In order to enroll, horses were to have a WCS ≥11 (see Table 4), weigh at least 200 kg, have a diagnosis of severe equine asthma with observation of at least one clinical sign for 14 days or more prior to enrollment, have history of at least two previous episodes of labored breathing at rest, and have history of improvement with appropriate treatment (for example, glucocorticoid administration, bronchodilator administration, and/or change in environment). Two hundred fifty-eight horses (134 AservoTM EquiHalerTM and 124 control) were included in the effectiveness analysis. Horses that were not fully dosed after enrollment due to the horse’s refusal to accept the device were included in the analysis as treatment failures.
Table 4: Weighted Clinical Score
| Parameter | Score: Description |
|---|---|
| Respiratory rate (breaths/minute) | 0: <16 bpm 1: 16-20 bpm 2: 21-25 bpm 3: 26-30 bpm 4: >30 bpm |
| Nasal Discharge | 0: None 1: Serous 2: Mucous 3: Mucopurulent |
| Nasal Flaring | 0: None 1: Present |
| Abdominal Lift | 0: None 1: Mild movement of abdomen and/or thorax and/or anus (with or without perceptible heaves line) 3: Pronounced movement of abdomen and/or thorax and/or anus (with or without perceptible heaves line) |
| Tracheal Sounds | 0: Normal 1: Increase in intensity 3: Mucus Movement |
| Bronchial Tones | 0: Normal 2: Audible ventral and/or dorsal sounds |
| Crackles | 0: None 2: Present |
| Wheezes | 0: None 2: Present |
| Cough | 0: None 1: Inducible by moderate pressure signal on larynx (only to be checked in the absence of intermittent or paroxysmal cough) 2: Intermittent 3: Paroxysmal |
A horse was considered a treatment success if there was a reduction in the WCS by at least 30% between Day 0/1 and Day 10, and had a Day 10 WCS ≤ 14. In this study, 52% of horses treated with Aservo™ EquiHaler™ vs. 33% of the horses treated with vehicle control had treatment success (p=0.0187), demonstrating the Aservo™ EquiHaler™ (ciclesonide solution for inhalation) is effective for the management of clinical signs associated with severe equine asthma in horses.
Phase 2 provided field safety and use information for repeated 10-day courses of treatment under conditions of use, including use with other appropriate treatment strategies. During Phase 2, 88 horses completed the full 100-day study. The most common reason for early withdrawal was perceived lack of effectiveness (n=7). Horses were to be prescribed a 10-day course of the Aservo™ EquiHaler™ when the WCS was ≥9.
Eight out of 108 did not receive additional inhaler devices. Of the 100 horses who qualified for retreatment, 52 (52%) qualified for one to three additional courses of treatment.
Two horses that were in the Aservo™ EquiHaler™ group during Phase 1 received nine additional courses of treatment in Phase 2, resulting in 100 days of exposure to Aservo™ EquiHaler™. Twenty-three (23%) horses received seven to nine courses of treatment during Phase 2. Concomitant medications and environmental changes intended for the overall management of severe equine asthma were allowed in Phase 2.
The most commonly prescribed concomitant medications included bronchodilators and antihistamines. Systemic antimicrobials were prescribed in six horses where the clinical signs of severe equine asthma were not adequately controlled, clinical signs worsened despite continued administration of Aservo™ EquiHaler™, or clinical signs of infection developed. Two horses received dexamethasone at early study withdrawal because their disease was insufficiently controlled. The most common environmental changes implemented during Phase 2 included soaking or steaming the hay, changing the forage source to either hay cubes or silage, changing the hay source (including stopping access to round bales), and increasing time out on pasture compared to time spent in the barn.

Please review the User Manual with horse owners to ensure they understand the entire contents. The User Manual includes important instructions regarding the proper handling and use of Aservo™ EquiHaler™. Demonstration of the activation, administration and storage of Aservo™ EquiHaler™ with the horse owner is highly recommended.

Store between 15 - 30°C. Protect from freezing. Use within 12 days of activation.

Aservo™ EquiHaler™ is supplied as one inhaler with a pre-inserted cartridge consisting of a plastic container crimped in an aluminum cylinder, which contains inhalation solution for 140 treatment actuations/puffs.
Boehringer Ingelheim Animal Health Canada Inc.
5180 South Service Road
Burlington ON L7L 5H4
Aservo™ and EquiHaler™ are trademarks of Boehringer Ingelheim Vetmedica GmbH, used under license.
Revised: 04/2020

Please read the following instructions carefully prior to first use of the Aservo™ EquiHaler™ (ciclesonide inhalation solution) which can be also found when using the URL info.equi-haler.com or the enclosed QR code.
The Aservo™ EquiHaler™ is an inhaler for horses. It contains sufficient inhalation solution for the complete course of the 10-day treatment. The inhaler cannot be refilled and should be disposed of 12 days after activation.

The Aservo™ EquiHaler™ is for left hand use only. While holding the Aservo™ EquiHaler™ with your left hand, hold and control your horse with your right hand.

Remove the Aservo™ EquiHaler™ from the outer carton.
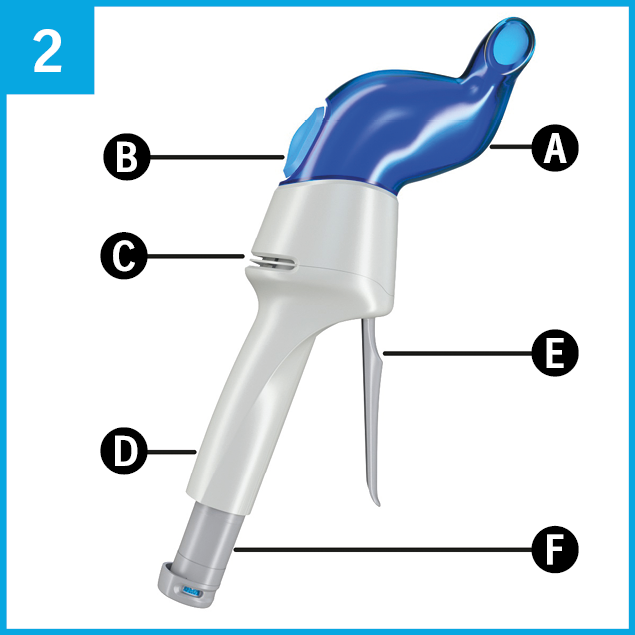
Familiarize yourself with the inhaler.
It consists of:
A Nostril adapter
B Breath indicator
C Air inlet
D Handle
E Prime and release lever
F Piercing element with fill indicator
In the following chapters, activation, preparation, administration, cleaning, and storage of the Aservo™ EquiHaler™ are described in detail.

Activate the Aservo™ EquiHaler™ only once prior to first use.
Use within 12 days of activation. Calculate 12 days from activation date and record this date on the device label on the “Use by _______” location provided.

To activate the Aservo™ EquiHaler™, the piercing element F must be inserted into the handle of the inhaler. Without pressing the lever E, put your right hand under the dark grey piercing element F…
Do not attempt to remove the piercing element; removal will cause significant damage to the device.

... and firmly push the piercing element F up completely into the handle D until you hear a click.
You will feel initial resistance when activating but continue to apply the force until the piercing element disappears entirely in the handle D and is no longer visible.
The Aservo™ EquiHaler™ is now activated, but not ready to use.
Excessive force can damage the device.

Preparation of the Aservo™ EquiHaler™ is required to ensure accurate initial dosing. Preparation is performed only once and consists of three (3) actuations (described below). The spray/mist will be fully visible after the third actuation.
When pressing the lever E of the Aservo™ EquiHaler™ for the first time, the lower part of the piercing element with the fill indicator F will become visible again. Do not push the piercing element back up into the device.

Each actuation/puff is a two-step process that results in release of the drug (one puff). It consists of the following steps (pictures 5 to 8):

Hold the Aservo™ EquiHaler™ upright in your left hand.

Step 1: Press the lever E until it touches the handle and a click can be heard.
Release the lever E allowing it to slide back into its starting position.

The display of the fill indicator in the piercing element is partially covered with a red flap.
Do not store the inhaler when the fill indicator is red as shown in picture 7 above.
Step 2: Use light pressure to press the lever E again only until you hear an audible click. The click will be heard after slight movement of the lever at which time the spray/mist will be released into the nostril adapter A.
For this step do not press the lever all the way to the handle.
Immediately release the lever and allow it to slide back into its starting position. The fill indicator display is now fully visible and the red flap no longer appears. The fill indicator displays the filling level in %.
Please note:
If the piercing element is accidently pushed completely into the handle again, it will automatically slide into the correct position the next time the Aservo™ EquiHaler™ is actuated.

Administration is intended for use in the left nostril only. The nostril adapter should remain in the nostril during the entire administration of the 8 or 12 actuations/puffs. If the nostril adapter slides out of the nostril during administration, please re-insert into the nostril again.

Hold the Aservo™ EquiHaler™ in your left hand. Make sure the air inlet C is not obstructed.
Stand on the left side of the horse so the horse’s head is next to your right shoulder.

Insert the nostril adapter A carefully into the horse’s left nostril, and gently rotate the inhaler into an upright position.

Ensure the nostril adapter is inserted in the nasal cavity.
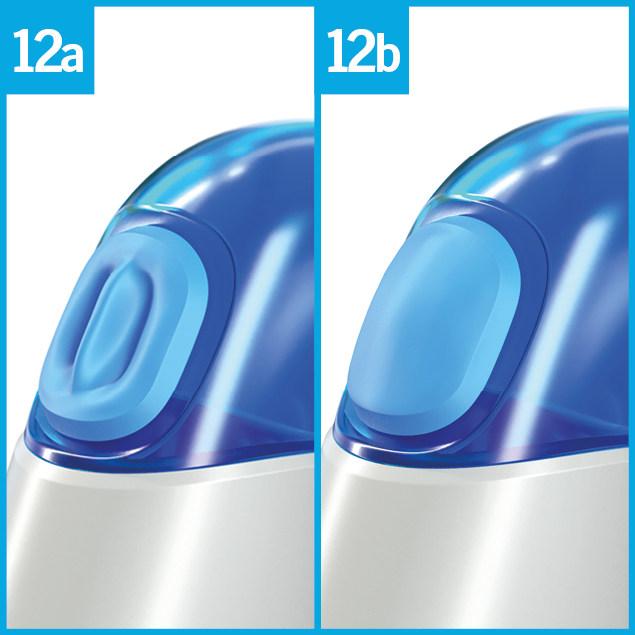
Observe the movement of the breath indicator B:
When the horse inhales, the breath indicator curves inwards (picture 12 a).
When the horse exhales, the breath indicator curves outwards (picture 12 b).
The optimum time for release is at the beginning of the horse´s inspiration when the breath indicator B begins to curve inwards (picture 12 a).
Please note: In order for the breath indicator to demonstrate when the horse inhales or exhales, the nostril adapter A must be correctly placed in the nostril and should have a snug fit.
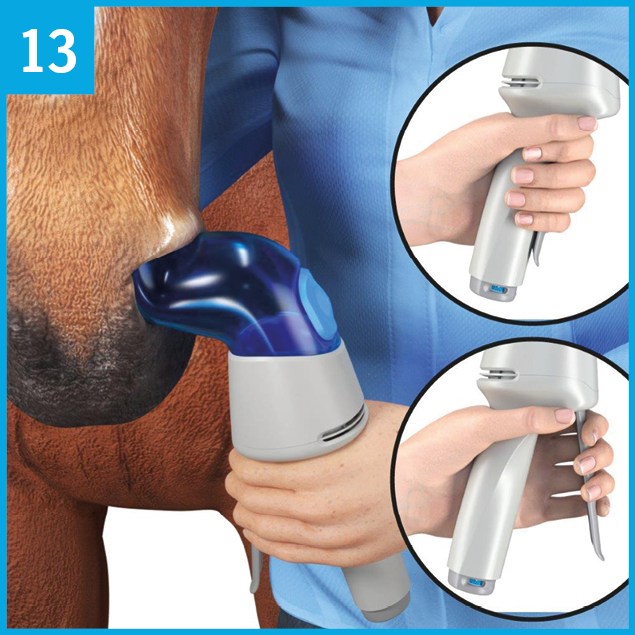
Once the Aservo™ EquiHaler™ is properly inserted, treatment can begin. Every actuation/puff should be performed following the two steps explained in pictures 6, 7, and 8.
Administer 8 actuations/puffs twice daily for the first five days.
Then administer 12 actuations/puffs once daily for the next five days, for a total of 10 days of treatment.
| Total treatment duration is 10 days | |
| Treatment days 1 to 5 |
8 actuations/puffs Twice daily |
| Treatment days 6 to 10 |
12 actuations/puffs Once daily |


The fill indicator shows the approximate percentage of actuations/puffs available in the inhaler.
The fill indicator should display 100% prior to first use, i.e. after the Aservo™ EquiHaler™ is prepared.
After Activation, store the inhaler with the fill indicator displaying blue/white only; do not store with the fill indicator on red.

The display of the fill indicator only moves after several actuations/puffs.
Please note the fill indicator is intended as a tool or gauge only.
After the completion of all doses or treatments, the display should be in the 0% position and the product should no longer be used. However, the lever will still work.
Manual recording of dosing is encouraged.
In the event the fill indicator does not appear to be functioning, it is important to continue to treat the horse as prescribed.
In addition, if the prescribed 10-day dosing has been completed do not continue to use if the fill indicator reads above 0%. If your horse continues to show clinical signs please contact your veterinarian.

Cleaning is only required on an as-needed basis. Wipe the outside of the nostril adapter with a damp cloth to remove foreign material, if needed.
Cleaning of the inner surface of the nostril adapter is not required unless nasal discharge or other foreign material is adhered to the inner surface. If it is necessary to clean the inner surface, remove the nostril adapter and rinse as described below.
Before cleaning, check that the fill indicator is blue/white. If it is red, press the lever E until the click is heard. This will ensure you do not accidentally release any spray. To avoid inhalation, hold the inhaler away from your body.

Twist and lift nostril adapter A from the handle D.
Store the handle in a clean and dry place.

Rinse the nostril adapter A only in clean running water. Do not use any brushes or cleaning products.
The nostril adapter is not suitable for the dishwasher.

The nostril adapter A must be air dried in an upright position for at least 4 hours.
Do not rub dry or heat.
Do not use equipment such as hair dryer, microwave, or heating element.

Once the nostril adapter A is dry, it should be reattached to the handle D by pushing it down firmly and twisting slightly until it slides into its place.
The nostril adapter A only locks in one position and should fit tightly into the handle.
The Aservo™ EquiHaler™ is now ready for the next use.

After Activation, store the inhaler with the fill indicator on blue/white only; do not store with the fill indicator on red.
Store at 15 – 30°C. Protect from freezing. Use within 12 days of activiation.